The McCarthy Lab aims to develop our understanding of the role of microbial communities (biofilms) play in infection progression and treatment failure. We also aim to identify new approaches to disrupt these bacterial communities.
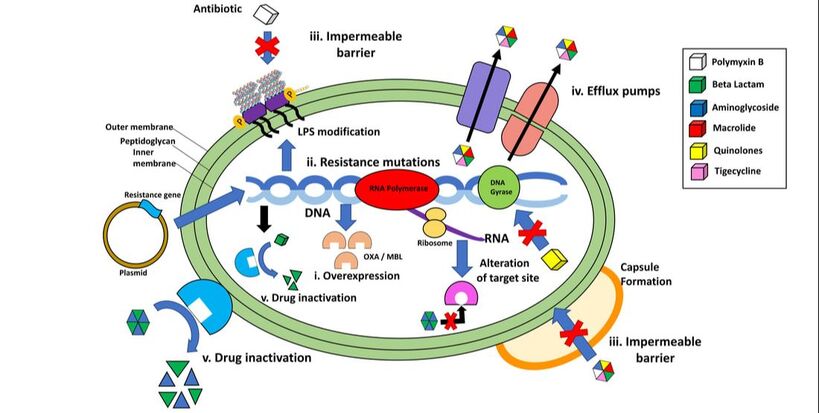
Acinetobacter baumannii survival strategies
In the last 30 years, Acinetobacter baumannii has risen from a relatively infrequent opportunistic pathogen to the top of the WHOs priority pathogen list due to its capacity to overcome antibiotic therapy. A large focus of our research team is centred on understanding how Acinetobacter baumannii regulates biofilm formation and desiccation tolerance and the role that these phenotypes play in treatment failure and hospital environment persistence. We aim to characterise the specific signal transduction cascades that regulates these survival phenotypes and explore their role in the evolution of resistance and tolerance to frontline and last resort antimicrobials.
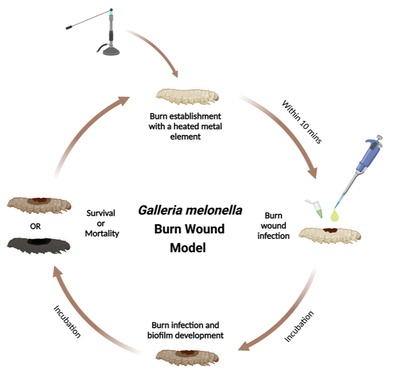
Wound infection
|
Wounds are associated with many types of medical intervention from caesarean sections to tooth extractions. The capacity for these wounds to become infected can severely complicate the treatment of the patient. Wound care and treatment also places a huge financial burden on global health care services. We are particularly interested in exploring how bacteria colonise and form biofilms within wounds. We use a range of in vivo and ex vivo platforms that we use to qualitatively and quantitatively determine the temporal dynamics of wound colonisation. We focus on characterising two Gram negative wound pathogens in particular, Pseudomonas aeruginosa and Acinetobacter baumannii.
|
Drug discovery
The antibiotic crisis and its potential impact on our global health care systems cannot be overstated. We are interested the identification and characterisation of compounds that have next generation antimicrobial activity. Next generation antimicrobials are compounds that do not kill bacteria but disables their ability to establish infection. This enables the immune system to successfully tackle the initial colonisation or can increase the efficacy of antibiotic therapy. We use a range of biosensors and reporter strains linked to a robotics platform to screen compounds libraries for next generation antimicrobial activity.
Plastic Bioremediation using Bacteria
Plastic pollution is a major environmental issue and represents a significant threat to our global ecosystem. Current strategies for the removal of plastic waste are ineffective and inefficient. Bacterial bioremediation represents a potential solution to the global plastic waste problem and numerous bacterial species and enzymes have been identified that can break down plastic waste. However, to date, the relative efficiencies of bacterial bioremediation of plastic waste are not conducive to upscaling to be a realistic solution to the plastic waste threat. We aim to leverage our understanding of bacterial community behaviour to maximise the bioremediation potential. We use a range of approaches to achieve including environmental sampling, targeted evolution, enzyme bioprospecting and enzyme optimisation.